+4 500
+1 700
+110
MIRIO is an innovative and collaborative web platform which optimizes the management of medical imaging in clinical trials, by facilitating the coordination between radiology and oncology.


They
They
Our
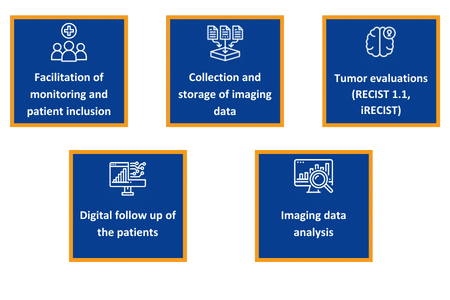
With MIRIO, we can manage and secure your medical imaging, making a difference in every step of your clinical trials. More than a collaborative platform, we provide turnkey and tailored-made medical services, giving you access to our hub of top-level radiologists.

Medical service
MIRIO has a hub of +500 senior French radiologists specialized in clinical trials

Technical
An unique platform for the whole medical team, used in healthcare establishments to improve their workflow

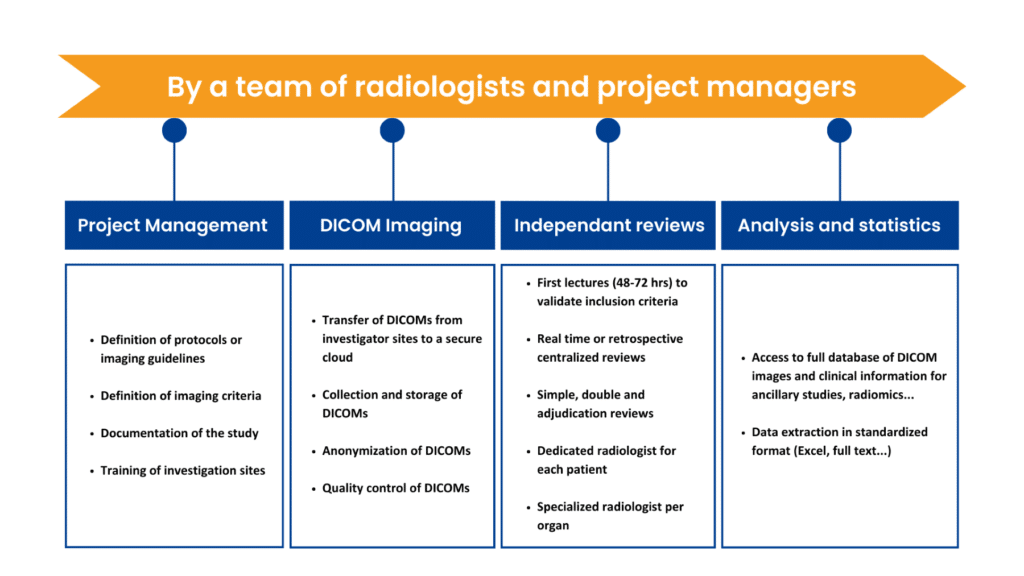
Project management
Quality, safety, ISO standards : we check all the boxes

Business model
Cut costs and save time by working directly with us

Regulatory
MIRIO is compliant with international health regulations

Medical device
Used in healthcare establishments to improve their workflow
Our
With MIRIO, we can manage and secure your medical imaging, making a difference in every step of your clinical trials. More than a collaborative platform, we provide turnkey and tailored-made medical services, giving you access to our hub of top-level radiologists.

Medical service
MIRIO has a hub of +500 senior French radiologists specialized in clinical trials

Technical
An unique platform for the whole medical team, used in healthcare establishments to improve their workflow

Project management
Quality, safety, ISO standards : we check all the boxes

Business model
Cut costs and save time by working directly with us

Regulatory
MIRIO is compliant with international health regulations

Medical device
Used in healthcare establishments to improve their workflow
Our
Our
MIRIO is used by 3 large french Cancer Research Centers and by AP-HP Paris.
It has also been deployed in more than 50 investigator sites.
Our
MIRIO is used by 4 large french Cancer Research Centers and by AP-HP Paris.
It has also been deployed in more than 50 investigator sites.